Borax tetrahydrate / sodium tetraborate (decahydrate) - OGR07 (ONLY WITH BUSINESS CARD)
OverviewIMPORTANT: Due to an adjustment in regulations this product now belongs to category 2 instead of category 1. As a result we are not allowed to sell this product to consumers and was our minimum order quantity changed to 5 kg.
ALWAYS note your BUSINESS CARD CODE, VAT number and COMPANY NAME when ordering. WIHTOUT this information we cannot deliver this product.
>>> request a Business Card free of charge
Description: Sodium tetra borate or borax is the sodium salt of boric acid. The substance is a white crystalline powder, which is solvable in water. A solution of 4% sodium tetra borate in water provides a pH of 9,2. A sodium tetra borate-solution has a buffering effect around this pH-level.
In nature it appears as the mineral 'borax', formally it is the decahydrate of sodium tetra borate. This name origins from the Persian burah. This is the name for the same mineral, which was known in ancient times and was used, amongst others, for the production of glass. The word traces back to the Middle-Persian burak. The name 'bor' was derived from the principal element of borax.
Sodium tetra borate is a poorly soluble salt in cold water, but solves well in warm water. After evaporation of a substance of sodium tetra borate, a vitreous layer arises, which, when adding to metal surfaces, avoids strong oxidation and improves the liquefying of metal. Therefore it is used as 'flux' during soldering. Sodium tetra borate can be converted to boric acid, by adding a strong acid like hydrogen chloride.
Use: A limited amount of use in cosmetics is allowed. It is used as decontaminant in powders and bath products.
In combination with glycerin (borax-glycerin), sodium tetra borate was used as antiseptic product in inflammations in the mouth (touch on).
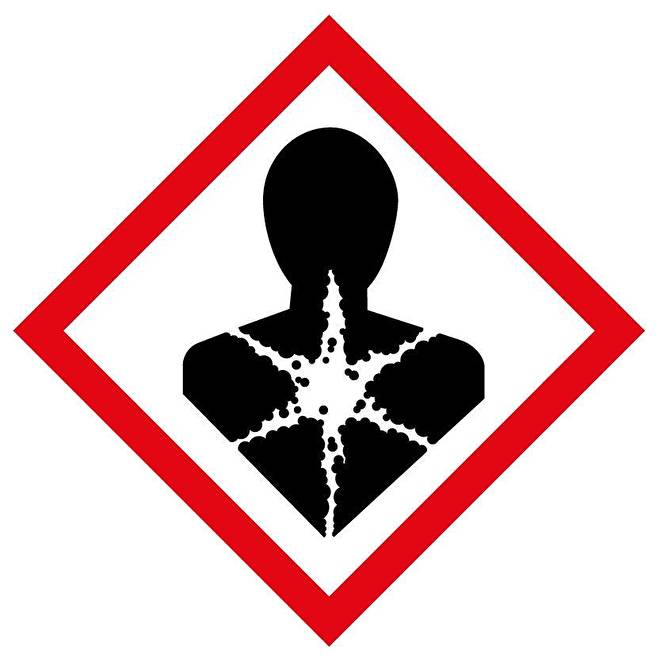
Pesticides against woodworm, which have a basis of sodium tetra borate and borix acid, are only used by professionals due to the toxicity. Because of the (moderate) toxicity sodium tetra borate is used as desinfectant as well. Since it is a salt, it is used by organic gardening to combat snails (salts impair the mucous membranes of snails). In a solution of 70 grams sodium tetra borate per bucket water (10 liter) it does not burn the roots of the plants, in contrast with sodium chloride. However, sodium tetra borate accumulates in the soil and can only be removed by rinsing. Therefore it is best to only use it once a year, since it can be harmful for plants in high concentrations.
Sodium tetra borate can be used in the analytical laboratory as standard solution for the volumetric measuerement of acid soluctions. It is a very appropriate primary standard to provide a standard solution of potassium permanganate. Natrium tetra borate was used for centuries as buffer fluid for eye drops. It has a poor antisceptic effect. Today it is not used as gargle anymore because of its toxicity.
Origin: Turkey (ETI Mine Works)
Chemical names: Borax decahydraat , Dinatriumtetraboraat decahydraat , Natriumboraatdecahydraat .
Purity: 99,90%
CAS: 1303-96-4
Weight: 5 kg - 25 kg
Wholesale Borax tetrahydrate sodium tetraborate:
Bulk 1 (250 kg) - Bulk 2 (1.000 kg)